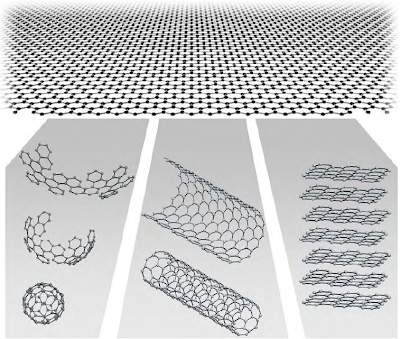
As I wrote here , physics Nobel Prize this year was awarded to Geim and Novoselov by the discovery of graphene. What makes graphene so special that it deserves this distinction?
Graphene is a crystalline form of carbon and one of the few materials that is two dimensional. That means it is very thin and which consists of only one plane of atoms. Like a sheet of paper, extends far into space in two dimensions than in the third dimension. The existence of a two-dimensional material by itself and was received with surprise news in the scientific world when it created the graphene for the first time in 2004. This is due to the fact that it had been shown theoretically that there should be a two-dimensional material. Due to fluctuations in its crystalline structure, resulting in the breaking of the 2D structure, any such fine material should be changed. But to the surprise of everyone if the graphene was stable when its synthesis was achieved. And so it is a very interesting material, although at first the interest was rather academic.
watermark Despite its appearance - the graphene is so thin that it hardly absorbs light and almost transparent - it is very robust. It is very difficult to break and is 200 times stronger than steel.
Another feature of graphene is that it is a good electronic conductor. This is because the organization of electrons in chemical bonds that form the carbon atoms in the plane. Carbon has four electrons that can participate in the formation of these links, but a carbon atom in graphene has only three neighbors. The three links to a carbon atom and its neighbors are called σ bonds (sigma) and are very stable. But since there are only three neighbors, only three electrons in each atom are attached to a link σ. The fourth electron forms a link called π (pi), but not sure which of the three neighbors. The electron is neither located nor attached to any atom. It is these electrons in π bonds responsible for the electronic conductivity of graphene. Like free electrons in a metal move without difficulty and represent the charge carriers in electronic conduction, the π electrons in graphene move easily, thus contributing to driving. Moreover, this complex behavior of electrons in σ and π bonds are responsible flat nature of graphene and its honeycomb appearance.
Graphene also has a wide variety of exceptional features such as high thermal conductivity and a host of interesting quantum effects. Does
graphene practical applications? Maybe in microelectronics and microprocessors such as transistors or integrated circuits, but for this to find everyday life path has yet to spend enough time. Alternatively graphene powder mix with a plastic socket. With a small amount of plastic insulating graphene can be transformed into a driver. And thanks to its transparency can be used as transparent electrode. We'll probably see many applications of graphene, and it therefore deserves the Nobel Prize.
For me the most curious is how it succeeds in producing graphene. Chemical method is not very developed, or need large and expensive instruments. In 2004, Geim and Novoselov used tape to peel layers of a graphite crystal. Paste these films on a silicon oxide surface is eventually in a sheet of graphene on silicon. Easy, right?
Graphene is a crystalline form of carbon and one of the few materials that is two dimensional. That means it is very thin and which consists of only one plane of atoms. Like a sheet of paper, extends far into space in two dimensions than in the third dimension. The existence of a two-dimensional material by itself and was received with surprise news in the scientific world when it created the graphene for the first time in 2004. This is due to the fact that it had been shown theoretically that there should be a two-dimensional material. Due to fluctuations in its crystalline structure, resulting in the breaking of the 2D structure, any such fine material should be changed. But to the surprise of everyone if the graphene was stable when its synthesis was achieved. And so it is a very interesting material, although at first the interest was rather academic.
watermark Despite its appearance - the graphene is so thin that it hardly absorbs light and almost transparent - it is very robust. It is very difficult to break and is 200 times stronger than steel.
Another feature of graphene is that it is a good electronic conductor. This is because the organization of electrons in chemical bonds that form the carbon atoms in the plane. Carbon has four electrons that can participate in the formation of these links, but a carbon atom in graphene has only three neighbors. The three links to a carbon atom and its neighbors are called σ bonds (sigma) and are very stable. But since there are only three neighbors, only three electrons in each atom are attached to a link σ. The fourth electron forms a link called π (pi), but not sure which of the three neighbors. The electron is neither located nor attached to any atom. It is these electrons in π bonds responsible for the electronic conductivity of graphene. Like free electrons in a metal move without difficulty and represent the charge carriers in electronic conduction, the π electrons in graphene move easily, thus contributing to driving. Moreover, this complex behavior of electrons in σ and π bonds are responsible flat nature of graphene and its honeycomb appearance.
Graphene also has a wide variety of exceptional features such as high thermal conductivity and a host of interesting quantum effects. Does
graphene practical applications? Maybe in microelectronics and microprocessors such as transistors or integrated circuits, but for this to find everyday life path has yet to spend enough time. Alternatively graphene powder mix with a plastic socket. With a small amount of plastic insulating graphene can be transformed into a driver. And thanks to its transparency can be used as transparent electrode. We'll probably see many applications of graphene, and it therefore deserves the Nobel Prize.
For me the most curious is how it succeeds in producing graphene. Chemical method is not very developed, or need large and expensive instruments. In 2004, Geim and Novoselov used tape to peel layers of a graphite crystal. Paste these films on a silicon oxide surface is eventually in a sheet of graphene on silicon. Easy, right?
For those seeking more detailed information on graphene, there are two texts by Andre Geim and Novoselov Konstantin (free access): The Rise of
Graphs: http://arxiv.org/abs/cond- mat/0702595
Graphene: Status and Prospects : http://arxiv.org/abs/0906.3799